Correct Answer

Answered by ExamLex AI
A nucleotide is composed of a nitrogenou...View Answer
Show Answer
Correct Answer
Answered by ExamLex AI
View Answer
Multiple Choice
Starch, dextran, glycogen, and cellulose are polymers of
A) amino acids.
B) fatty acids.
C) glucose.
D) acids.
E) nucleic acids.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an amino acid contained a hydrocarbon as its side group, in which of the following categories could it be appropriately designated?
A) polar
B) basic
C) hydrophilic
D) acidic
E) nonpolar
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the following reaction: HCl + NaHCO3 -NaCl + H2CO3
A) dehydration synthesis reaction
B) hydrolysis reaction
C) reversible reaction
D) ionic reaction
E) exchange reaction
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule is composed of a chain of amino acids?
A) nucleic acid
B) lipid
C) protein
D) carbohydrate
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type of molecule contains - NH2 groups?
A) nucleic acid
B) protein
C) triglycerides
D) carbohydrate
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Figure 2.1
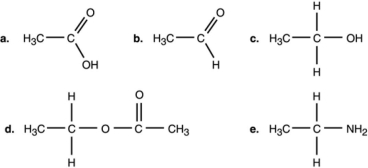 -Which compound in Figure 2.1 is an organic acid?
-Which compound in Figure 2.1 is an organic acid?
A) a
B) b
C) c
D) d
E) e
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Table 2.1 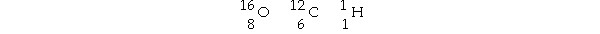 -Using the information in Table 2.1, calculate the molecular weight of ethanol, C2H5OH.
-Using the information in Table 2.1, calculate the molecular weight of ethanol, C2H5OH.
A) 33
B) 34
C) 96
D) 46
E) The answer cannot be determined.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
A basic solution is expected to contain more hydrogen ions than hydroxyl ions.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Any compound that contains carbon is only considered to be organic.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you viewed one single protein using a microscope, you would observe multiple structures.
A) secondary
B) tertiary
C) primary
D) primary and secondary
E) secondary and tertiary
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Individual covalent bonds are stronger than individual ionic bonds.
B) False
Correct Answer

verified
Correct Answer
verified
Essay
A scientist claims that when a protein is denatured, it can be expected that its secondary structure will more likely be retained when compared to all other levels of protein structure structures. Do you agree? Explain.
Correct Answer

Answered by ExamLex AI
I do not agree with the scientist's clai...View Answer
Show Answer
Correct Answer
Answered by ExamLex AI
View Answer
Multiple Choice
Which of the following is a base?
A) C2H5OCOOH -H+ + C2H5OCOO-
B) NaOH -Na+ + OH-
C) C2H5OH
D) H2O -H+ + OH-
E) H2CO
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which are the primary molecules making up plasma membranes in cells?
A) nucleic acids
B) carbohydrates
C) lipids
D) proteins
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radioisotopes are frequently used to label molecules in a cell. The fate of atoms and molecules in a cell can then be followed. Assume Saccharomyces cerevisiae is grown in a nutrient medium containing the radioisotope 35S. After a 48- hour incubation, the 35S would most likely be found in the S. cerevisiae's
A) water.
B) nucleic acids.
C) proteins.
D) carbohydrates.
E) lipids.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the type of bond between the hydrogen of one molecule and the nitrogen of another molecule?
A) ionic bond
B) hydrophobic bond
C) hydrogen bond
D) disulfide bond
E) covalent bond
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two glucose molecules are combined to make a maltose molecule. What is the chemical formula for maltose?
A) C12H22O11
B) C12H24SO12
C) C12H23O10
D) C6H12O6
E) C3H6O3
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Figure 2.3
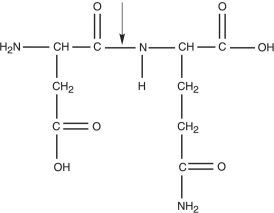 -What kind of bond is at the arrow in Figure 2.3?
-What kind of bond is at the arrow in Figure 2.3?
A) double covalent bond
B) hydrogen bond
C) peptide bond
D) disulfide bridge
E) ionic bond
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The formation of ADP from ATP can be defined as a hydrolytic reaction.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 58
Related Exams